Early Release – Limited Outbreak of Highly Pathogenic Influenza A(H5N1) in Herring Gull Colony, Canada, 2022 – Volume 29, Number 10—October 2023 – Emerging Infectious Diseases journal

[ad_1]
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliations: Yale University, New Haven, Connecticut, USA (L.U. Taylor, C.B. Ogbunugafor, A.J. Ayala); Canadian Wildlife Service, Dartmouth, Nova Scotia, Canada (R.A. Ronconi); Environment and Climate Change Canada, Dartmouth (R.A. Ronconi); University of Guelph, Guelph, Ontario, Canada (H.A. Spina); University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada (M.E.B. Jones); Canadian Wildlife Health Cooperative, Charlottetown (M.E.B. Jones)
Highly pathogenic avian influenza (HPAI) viruses pose a near-term threat to commercial poultry and a long-term risk for human pandemics (1,2). Recent outbreaks of HPAI A(H5N1) virus have also caused mass mortality events in vulnerable seabird populations (3). Because outbreaks are difficult to predict, knowledge of HPAI in wild birds is often limited to cross-sectional surveillance or post hoc records of mass mortality events (4–6).
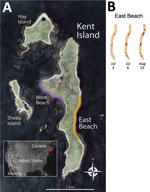
Beginning in December 2021, an HPAI H5N1 virus strain spread from Eurasia into Canada, subsequently infecting wild, commercial, and backyard bird populations across North America (4) (https://www.usgs.gov/centers/nwhc/science/distribution-highly-pathogenic-avian-influenza-north-america-20212022). During summer 2022, we studied the life history of American herring gulls (Larus argentatus subspecies smithsonianus) at the Kent Island breeding colony in New Brunswick, Canada (Figure 1). Thus, we had an unusual opportunity to monitor emerging disease symptoms and deaths in a wild population. We report timelines, clinical details, and epidemiologic observations from a laboratory-confirmed HPAI outbreak that caused a relatively low death rate within a seabird colony.
We monitored herring gulls on Kent Island (latitude 44.5828°N, longitude 66.7568°W; Figure 1). Gulls nest across the ≈100-ha island and on adjacent Hay and Sheep Islands (Figure 1). Herring gulls on Kent Island generally migrate north from eastern North America in early May, lay eggs in mid-May, hatch chicks in mid-June, and fledge chicks in August (7,8).
During June 1–August 15, 2022, we surveyed the main study area on East Beach (Figure 1) 1–3 times/day, conducting full census counts, monitoring disease symptoms, and individually marking carcasses. Other areas of Kent Island were surveyed on an intermittent schedule (Table 1). We assumed that all generally intact adult carcasses were virus-induced deaths because sudden deaths of adult birds are rare in breeding colonies. Because injuries and deaths are common among chicks, we were unable to assess virus-induced deaths in chicks except for suspected cases C1–C3 (Appendix).
We did not observe illness in the colony during a preliminary visit to Kent Island (May 24–27). On the morning of June 27, we spotted a lethargic adult herring gull on East Beach that died that afternoon (Table 2; Figure 2). Disease symptoms and deaths spiked at 9 deaths during July 4–8 (Figure 2). We observed 9 more deaths that accumulated more slowly through August 15; a final check on September 2 revealed only 1 new carcass. The total number of East Beach cases was 25, resulting in 22 confirmed deaths (4.2% site mortality; Tables 1, 2). Daily checks of West Beach for part of the summer showed a similar timeline and effect as that observed on East Beach (Tables 1, 2). Total carcass counts across Kent, Sheep, and Hay Islands indicated a <10% mortality rate (Table 2).
During the summer 2022 breeding season, colony populations declined beginning in July (Figure 2). We assume that gulls exited the breeding colony because of normal seasonal phenology (8) rather than off-site deaths. Boat surveys of the surrounding Grand Manan archipelago (mid-June, mid-July) noted only 3 dead adult herring gulls in the water, and no mass mortality was reported on nearby beaches (1 dead HPAI virus–positive herring gull was found on Grand Manan on July 4; https://cfia-ncr.maps.arcgis.com/apps/dashboards/89c779e98cdf492c899df23e1c38fdbc). Censuses in June 2023 confirmed that the Kent Island herring gull population had returned for another breeding season (mean 4,290 herring gulls).
We collected case descriptions, images, and videos of herring gull adults and chicks from Kent Island (Appendix). Putative HPAI clinical signs in herring gulls matched those observed after experimental inoculations of HPAI H5N1 in related species (9,10). Neurologic symptoms progressed from lethargy and drooped wings to incoordination, head tremors, torticollis, and immobility over the course of hours or days. During the peak of the outbreak, dozens of additional birds displayed putative minor symptoms (e.g., slumped postures, hesitancy to fly) that were difficult to track and could not be linked to subsequent death. One bird manifesting severe neurologic distress apparently recovered within hours (case 27).
We collected 3 carcasses of adult symptomatic birds (case 8, case 20, and 1 euthanized bird in southwest Kent Island on July 15) along with 3 chicks (cases C1–C3). Carcasses were collected under Canadian Wildlife Service permit no. SS2506 (to R.A.R.). All 3 adults and 1 chick (case C2) tested positive for a Eurasian strain of HPAI H5N1 virus (Appendix).
All sick or dead adult gulls throughout June and July were >4 years old according to plumage, matching the usual minimum breeding age for the species (Table 2) (8). Plumage-based censuses suggested 3%–6% of colony birds were 1–3 years of age (data not shown). Those younger birds were not breeding, and only 2 were found dead on East Beach later in the summer (Table 2). From 16 fully-tracked cases (Table 2) and surveys conducted 1–3 times/day, we showed the mean time (+SD) from first seen sick to last seen alive was 7.8 +15.0 hours; the mean time from first seen sick to found dead was 20.9 +14.9 hours.
We calculated the basic reproduction number (R0) by using daily East Beach incidence data (June 1–August 15), gamma-distributed generation times from poultry data (4.8 +0.58 days) (11), and the exponential growth rate method from the R package R0 (12). Overall R0 was 1.02 (95% CI 0.95–1.11). R0 was 8.23 (95% CI 3.97–21.11) if estimated from the rising incidence period (June 1–July 6) but fell to 0.84 (95% CI 0.64–1.07) if estimated from the falling incidence period (July 7–August 15).
HPAI was suspected or confirmed in 4 other species breeding on Kent Island (Appendix): great black-backed gulls (Larus marinus), Canada geese (Branta canadensis), common eiders (Somateria mollissima), and American crows (Corvus brachyrhynchos). Unlike the mostly intact gull carcasses on Kent Island (Appendix), many carcasses on Hay Island were partially consumed. Likely predators or scavengers were great black-backed gulls and bald eagles (Haliaeetus leucocephalus). Beginning in July, we noted gray seals (Halichoerus grypus) loitering offshore at East Beach. Seals rarely interacted with adult seabirds but harassed herring gull chicks paddling from shore.
A Eurasian lineage of HPAI H5N1 virus swept through the Kent Island herring gull colony starting in late June 2022. The outbreak appeared to slow within weeks (Figure 2) and resulted in <10% apparent colony mortality rate (Table 1). Low carcass disturbance (Appendix Table 1) and disease resistance or recovery (case 27) might have limited HPAI virus infections in the gulls. Furthermore, our islandwide censuses suggest 2022 population sizes were <25% of historical size across the same island area (Table 1) (13). Low densities might have reduced intraspecific transmission by limiting social interactions with infected conspecifics. However, we observed possible interspecific exposure routes through cohabitation (e.g., common eiders), predation/scavenging (e.g., bald eagles), and interactions between chicks and marine mammals (e.g., gray seals). Those pathways are consistent with global HPAI virus transmission between populations, including recent spillover events in mammals (14,15).
The current understanding of HPAI virus transmission in wild birds involves circulation in migratory waterfowl or roving gulls (6) and mass mortality events within seabird colonies (3,5). Our study suggests that limited outbreaks in seabird colonies could play an important role in HPAI transmission chains. Post hoc surveillance of mass mortality is insufficient if seabird colonies can circulate HPAI without mass mortality. Therefore, we propose that more proactive monitoring of seabirds for HPAI virus infections will be critical for guarding commercial poultry (1), averting potentially catastrophic zoonotic transmission (2), and protecting vulnerable seabirds, including gulls.
Mr. Taylor is a PhD candidate in the Department of Ecology and Evolutionary Biology at Yale University. He studies how social development influences, and is influenced by, the life history evolution of birds, with a focus on delayed reproduction and delayed plumage maturation in lekking manakins and colony-breeding gulls.
We thank Patricia Jones, Ian Kyle, Sarah Mueller, Sarah Dobney, and the students, staff, and faculty at the Bowdoin Scientific Station on Kent Island for fieldwork support; Yen-Hua Huang for providing helpful comments on the manuscript; and the Atlantic Region Canadian Wildlife Health Cooperative for assistance with laboratory testing.
Confirmatory laboratory test results were obtained from Canada’s interagency surveillance program for avian influenza viruses in wild birds, a partnership that includes Environment and Climate Change Canada, the Canadian Food Inspection Agency, Canadian Wildlife Health Cooperative, and other federal, provincial, territorial, indigenous, and academic partners involved in wildlife, domestic animal, and human health (see website for full list of partners: https://cfia-ncr.maps.arcgis.com/apps/dashboards/89c779e98cdf492c899df23e1c38fdbc).
This work was supported by the National Science Foundation graduate research fellowship program (no. DGE1752134 to L.U.T.), National Science Foundation postdoctoral fellowship in biology (no. 2010904 to A.J.A.), the W.R. Coe Fund from Yale University, and Environment and Climate Change Canada.
This is contribution no. 293 of the Bowdoin Scientific Station.
[ad_2]
Source link