Evaluating WHO’s Hemophilia Treatment Choices: Patient Safety, Access Challenges

[ad_1]
Red Blood Cells | Image Credit: Design Cells – stock.adobe.com
Recent decisions made by the World Health Organization (WHO) regarding the inclusion and categorization of treatments for hemophilia in its Essential Medicines List (EML) have sparked concerns among patients and health care providers.
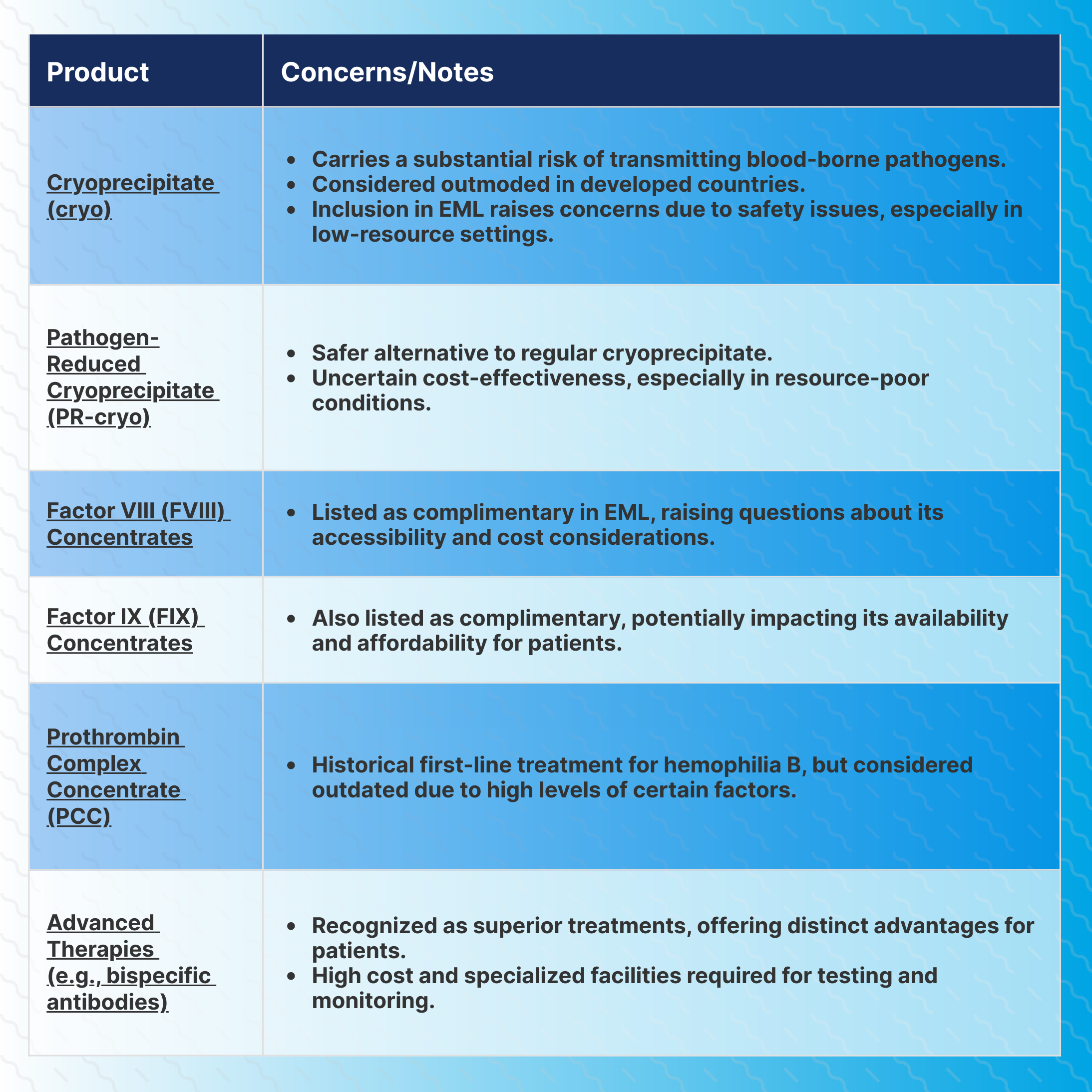
One of the primary concerns revolves around the characterization of cryoprecipitate (cryo), which is not pathogen-reduced (PR), as an alternative modality for treating hemophilia, despite its substantial risk of transmitting blood-borne pathogens to patients.
The decision to include cryo in the EML raises questions about patient safety and the potential transmission of infections in countries where blood safety standards might not be as stringent, according to a commentary article published in Haemophilia by Albert Farrugia, PhD, University of Western Australia.
PR-cryo: Affordable, Precise Treatment
Cryo was the first therapeutic product available for treating bleeding disorders and has long been utilized for conditions like hemophilia A, von Willebrand’s disease, and fibrinogen deficiency. However, in developed countries, the unmodified version is considered outdated, with specific concentrates of the deficient proteins being the preferred mode of treatment.
The inclusion of cryoprecipitate [pathogen-reduced] (PR-cryo) in the EML is especially relevant for resource-poor conditions. PR-cryo, specified for Factor VIII (FVIII), von Willebrand Factor, and fibrinogen, offers a more affordable and accurate dosing option.
Pooled and aliquoted from individual cryoprecipitates, PR-cryo ensures the potency of the relevant factors, allowing for precise and effective treatment. This method is pivotal in areas where resources are limited, ensuring patients receive the necessary care without the burden of excessive costs.
The WHO Global Status Report on Blood Safety and Availability highlights a significant disparity in blood safety between low- to middle-income countries (LMIC) and high-income countries (HIC). It’s reported that LMICs face a much higher prevalence of blood-borne viruses, posing a considerable risk to patients relying on blood products for treatment.
The commentary discusses how the availability of several commercial versions of PR-cryo might impact the cost-effectiveness of the therapeutic modality. Coagulation factor concentrates, especially for hemophilia A, have become more affordable in recent years, benefiting developing countries.
However, Farrugia referenced a recent report that suggests domestically sourced PR-cryo might offer a cheaper alternative demonstrating the importance of ongoing research and international cooperation to ensure the most cost-effective solutions reach those in need.
Categorization of Hemophilia Treatments
The decision to categorize FVIII and factor IX (FIX) concentrates as complementary rather than core medicines has raised concerns within the medical community. While these concentrates are recognized as the preferred route of replacement therapy for hemophilia A and B, their listing status might be influenced by cost perceptions, leading to reservations among health care providers.
Patients with hemophilia B, in particular, could experience disadvantages if a core-listed medicine is not specified, calling for careful reconsideration of these classifications to ensure equitable access to essential treatments.
Reconsidering Alternative Treatments
Another area of concern was noted from the inclusion of Prothrombin Complex Concentrate (PCC) as an alternative treatment to FIX concentrates for hemophilia B. Despite its historical use, evidence-based practice has shown that PCC does not align with the advancements made in hemophilia care.
This decision to list PCC as an alternative treatment, Farrugia wrote, contradicts established medical practices and warrants urgent reevaluation to ensure patients receive the best and safest treatments available.
The commentary acknowledged the WHO’s recognition of the value of blood products in hemophilia treatment but stated there is a pressing need for a comprehensive review of the EML in the context of evolving medical advancements. Incorporating safer, more effective treatments while addressing the economic challenges faced by low-income countries should be at the forefront of decision-making.
Reference
Farrugia, A. The World Health Organisation’s list of essential medicines and haemophilia treatment products. Haemophilia. 2023; 1-3. https://doi.org/10.1111/hae.14879
[ad_2]
Source link
