Predictors of Viral load and Medication Adherence

[ad_1]
Background
HIV/AIDS is one of the major global health problems for both developed and developing countries.1 About 37.7 million people worldwide are living with HIV, with 36.0 million of them being adults.2
Eastern and Southern Africa have been impacted the hardest by HIV. In comparison to other parts of the world, Sub-Saharan African countries have the most severe effects of HIV/AIDS.3
Ethiopia is one of the most affected countries in Sub-Saharan Africa, with a large number of HIV/AIDS patients.4 In Ethiopia, people living with HIV (PLHIV) was estimated in 2020 to be 620,000 people. There were 580,000 adults.5
Amhara region is one of the regions in Ethiopia severely hit by the disease.6 In the Amhara region, including the catchment area of Felege Hiwot Comprehensive Specialized Hospital (FHCSH), many antiretroviral therapy (ART) users did not seriously adhere to their prescribed medication.7 Due to this, most of the ART users in the region have less than 95% of the prescribed medication and over 30% of the patients are poor adherents.8
The gold standard laboratory measure for predicting disease progression and/or treatment outcomes is viral load.9 Treatment outcomes are monitored by World Health Organization (WHO) clinical staging, immunological (CD4 T-cell count), and routine viral load testing. In comparison to viral suppression, immunological and clinical monitoring has a lower positive predictive value for detecting treatment failure.10 According to the Ethiopian Federal Ministry of Health (FMOH), the first viral load determination for people living with HIV should be done 6 and 12 months after starting ART, and then every year after that.11
The increment of viral load was affected by several factors including baseline CD4 cell count, WHO clinical stage, functional status, medication adherence, smoking status, and baseline regimen.12
The WHO defines medication adherence as the extent to which a person takes medications properly.13 In HIV-positive patients, good adherence to antiretroviral medication suppresses viral load, reconstitutes the immune system, and reduces opportunistic infections. However, in developing countries like Ethiopia, adherence to ART is still a challenge.14
Repeated measures of viral load would be a superior method for identifying treatment failure than a single measurement, which might lead to treatment failure misinterpretation.15
To the authors’ knowledge, no other study has been conducted to assess predictors affecting these two outcomes (viral load and medication adherence) jointly around the study area. Therefore, this study was undertaken with the objective to identify some common socio-demographic and clinical predictors affecting the two responses, namely the status of viral load and medication adherence for adult HIV infected patients under treatment at FHCSH.
Methods
Study Area and Study Design
The study was conducted in northwest Ethiopia, Amhara region (FHCSH and its catchment area). A retrospective study design was conducted in the current investigation. The source of data used for this investigation was a secondary data source.
Sample Size and Sampling Procedure
In this study, to calculate the sample size, first the estimated proportion of successes (good medication adherence) was computed; in a dichotomous outcome variable (yes/no) in a finite population the Cochran’s formula was used. In the current study, the response variables were dichotomous in nature (Viral suppuration or not and medication adherence or not). Hence, the formula for determining sample size was determine using Cochran`s formula as given by:
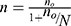 , where
, where  and N is the target population size, the target population was the number of adult patients whose age above 18 living with HIV/AIDS on ART follow-up in the study period.
and N is the target population size, the target population was the number of adult patients whose age above 18 living with HIV/AIDS on ART follow-up in the study period.  is the value from standard normal distribution reflecting the confidence level, 95% Confidence interval. Since
is the value from standard normal distribution reflecting the confidence level, 95% Confidence interval. Since  and d is the marginal error, p is the sample proportion of good medication adherence.
and d is the marginal error, p is the sample proportion of good medication adherence.
If  is negligible (ie, <0.005), then no is a satisfactory approximate to the sample size n≈no (Cochran, 1977).16 Therefore, the sample size was determined by using single proportional formula. With 5% marginal error and 95% confidence interval of certainty and considering the possible missing records (less than two follow-ups) to compensate them, 5% of the total sample size n was added. Hence the total population N=1,126 and 95% of confidence interval, finally the total sample size was n=281. This indicates that Cochran’s formula was employed for this investigation. Of the 1,126 adult people living with HIV (PLHIV) aged above 18 and under treatment in the hospital using ART were followed-up from June 2017 to June 2021, a random sample of 281 were including in this study. The samples were selected using simple random sampling. To compensate the excluded patients (those individuals with incomplete visiting times), 5% additional samples were considered.
is negligible (ie, <0.005), then no is a satisfactory approximate to the sample size n≈no (Cochran, 1977).16 Therefore, the sample size was determined by using single proportional formula. With 5% marginal error and 95% confidence interval of certainty and considering the possible missing records (less than two follow-ups) to compensate them, 5% of the total sample size n was added. Hence the total population N=1,126 and 95% of confidence interval, finally the total sample size was n=281. This indicates that Cochran’s formula was employed for this investigation. Of the 1,126 adult people living with HIV (PLHIV) aged above 18 and under treatment in the hospital using ART were followed-up from June 2017 to June 2021, a random sample of 281 were including in this study. The samples were selected using simple random sampling. To compensate the excluded patients (those individuals with incomplete visiting times), 5% additional samples were considered.
Inclusion Criteria
HIV/AIDS patients whose age was above 18 years old and attended a minimum of two follow-up visits for ART treatment for refilling their prescription, and who were initiated on ART from June 2017 to June 2021 were included in this investigation.
Variables Under Investigation
The two response variables considered for this study were viral load count and medication adherence (medication adherence and medication non-adherence) for HIV patients under treatment.
Covariates associated with viral load and medication adherence among patients living with HIV comprise baseline, socio-demographic and clinical variables such as age in years, gender (male, female), residence (urban, rural), marital status (single, married, divorced, and widowed), level of education (no formal education, primary, secondary, and tertiary), religion (Muslim, orthodox, and other), Social support (yes, no), substance use (yes, no), weight, TB status (negative, positive), WHO stage (stage I, stage II, stage III, and stage IV), functional status (ambulatory, bedridden, and working), body mass index (underweight, normal, and overweight), opportunity of infection (yes, no), hemoglobin in grams per deciliter, CD4 cell count in cells per cubic millimeter, ART regimen (AZT,3TC,NVP,AZT,3TC,EFV,TDF,3TC,EFV,TDF,3TC,NVP and TDF,3TC,DTG), and disclosure status (disclosed, not disclosed).
Measurement of Medication Adherence
Medication adherence is measured by using pill counts and counted by pharmacists at the treatment session. Patients are asked to bring all medication bottles and unused pills to each clinic visit, but they are not told that the returns are to be counted.17 Adherence at every visit for all the drugs was calculated as:18
Statistical Models
The model to analysis longitudinal data is a generalized linear mixed effect mode. This model includes both fixed effect and random effect.
In this investigation before construction of joint model analysis, longitudinal separate models were applied to identify the predictors that have a significant effect on the response variable separately and to compare the separate and joint models of longitudinal responses. To apply the potential covariance structure and parsimonious random effect models, information criteria (AIC and BIC) were employed.
Results
The descriptive part of the study deals with the study variables and their categories, considering the response and predictors, as shown in Table 1. Table 1 displays that, among the total 281 HIV infected patients that were considered in this study, about 58.72% were female and about 41.28% were male. About 36.3% lived in a rural area and about 63.7% lived in an urban area. About 87.9% were Orthodox religious followers and about 8.54% were Muslim religious followers.
 |
Table 1 Frequency Distribution for Covariates |
Regarding marital status, the majority of the patients, about 41.99%, were married, about 27.40% were single, and about 21.35% were divorced. About 29.54% had no formal education and 24.91% had secondary education level. Most of the patients (55.16%) receivedsocial support and about 44.48% did not get social support. The majority of the patients (about 73.31%) were free from substance use and about 26.69% were not free from substance use. About 49.82% of patients had disclosed their HIV status to their partner, and 50.18% had not.
The mean age of HIV infected patients at baseline was 34 years old with a standard deviation of 11.1. The mean weight of HIV infected patients at baseline was 54 kg with a standard deviation of 10.6 kg. The mean hemoglobin level of HIV infected patients at baseline was 13.3 gm/dL, with a standard deviation of 2.5 gm/dL. The median CD4 cell count of all adult patients was 145 gm/mm3 with the lower and upper quartiles (112 gm/mm3, 125 gm/mm3).
Exploratory Data Analysis
Exploring Individual Profile Plot for Patient’s Viral Load
The viral load trend in Figure 1 shows that some patients had erratic viral load patterns, some patients had a slowly decreasing rate, and some patients had a slowly increasing rate. Hence, there is evidence of between-subject variability as well as within-subject variability. Patients had different viral load values at the start and possibly different values over time.
 |
Figure 1 The individual profile plot of patient’s viral load. |
Exploring Mean Profile for Patient’s Viral Load
The mean profile of viral load was a high rate at the first assessment then it started to decrease slowly over time.
Probability Plot for Patient’s Medication Adherence
The probability plot indicated in Figure 2 shows that the probability of the patient to be adherent to medication over time was increased.
 |
Figure 2 Probability plot of medication adherence over visiting time. |
The Separate Longitudinal Analysis for Viral Load
For the generalized linear mixed-effect model to be valid, the covariance structure among repeated measures must be modeled properly.
In Table 2, the model that contains an unstructured covariance structure was the best fit model to the current data compared to the other remaining covariance structure, as it had the smallest values of AIC and BIC as compared to others.
 |
Table 2 Comparison of Covariance Structure for GLMM for the Response Viral Load |
According to the result of Table 3, the visit time, marital status, substance use, medication adherence, WHO clinical stage, initial ART regimen, baseline CD4 cell count, and baseline hemoglobin were statistically significant (p<0.05) predictor variables for viral load.
 |
Table 3 Parameter Estimation for Generalized Linear Mixed Effect Model for the Response Viral Load |
The random effect estimates indicated that intercepts and slopes were significantly varied, which suggests that there was significant considerable variation from visit to visit in adult HIV/AIDS patients (p<0.05). The amount of variability among patients at intercept was 3.29 and due to the effect of time per month in each visit was 0.004. Hence, the correlation between intercept and slope was −0.766. Table 3 indicates that as visiting time (in months) of patients increased by one unit the log of expected viral load was decreased by 0.025 copies/mL (p<0.0001) given that other predictors were constant.
The Separate Longitudinal Analysis for Medication Adherence
Table 4 revealed that an unstructured covariance structure was the best fit model for the current data due to the smallest values of AIC and BIC as compared to others.
 |
Table 4 Comparison of Covariance Structure for the Response Medication Adherence |
A univariable generalized linear mixed-effect model was fitted to select potential explanatory variables that were included in the multivariable generalized linear mixed-effect model for medication adherence. The explanatory variables significant at a 25% level of significance in the univariable analysis can be candidates for multivariable analysis in the purposeful variable selection method. Hence, gender, age, visit time, social support, substance use, disclosure, baseline CD4 cell count, WHO clinical stage, TB status, and hemoglobin were candidates for a multivariable generalized linear mixed-effect model for the outcome medication adherence for 25% level of significance.
Table 5 shows that visit time, social support, substance use, baseline CD4 cell count, and the interaction of time by substance use were statistically significant predictor variables for medication adherence at the 5% of level of significance.
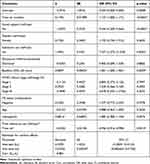 |
Table 5 Parameter Estimation for Generalized Linear Mixed Effect Model for the Response Medication Adherence |
The random effect estimates indicated that intercepts and slopes were significantly variables, which suggests that there was a significant considerable variation in patient’s medication adherence from visit to visit in adult HIV/AIDS patients (p<0.05). Table 5 indicates that as the visit time (in months) of adult HIV/AIDS patients increased by one unit the probability of good medication adherence was increased by 0.119 (p<0.0001) given other predictors were constant.
Joint Model Analysis for Viral Load and Medication Adherence
To assess the association between count and binary longitudinal outcomes, the joint generalized linear mixed model was fitted. The correlation between the two responses is specified through the random effect. A direct specification of joint distribution for both outcomes using a mixed effects model was developed. The observed correlation between the two responses arises from the association of random effects.19
Separate and joint model were compared based on standard error computed in the two models for significant predictors. From the result of separate and joint models, the joint model had a smaller standard error as compared to the separate models. This means that the joint model was more precise than the separate models. And also, there was a highly significant correlation between the two longitudinal sub-models in joint modeling analysis. Therefore, the joint model performed better than the separate models.
Table 6 shows that visit time, substance use, baseline CD4 cell, and baseline hemoglobin were significant predictors associated with viral load and medication adherence jointly.
 |
Table 6 Parameter Estimation for Joint Modeling of Viral Load and Medication Adherence |
In addition, the interaction of visit time by substance use also associated with both outcomes. In joint model analysis, the predictors’ WHO clinical stage and marital status were significantly associated with viral load. And only social support had a significant predictor associated with medication adherence.
According to the joint model analysis, the result interpreted that, for a patient’s visit time (in months) increase by one unit, the expected viral load for adult HIV infected patients was decreased by 0.053 copies/mL (p=<0.01) and the estimated odds of being medication adherent were increased by 10.4% (p=<0.0001) given other predictors were constant.
The expected viral load for non-substance user adult HIV infected patients was decreased by 1.609 copies/mL (p=<0.0001) as compared to substance user adult HIV infected patients. However, the estimated odds of medication adherence of non-substance user adult HIV infected patients was 6.458-times that of substance users (p=<0.0001).
As baseline CD4 cells (cells/mm3) increased by one unit, the expected viral load of patients under treatment was decreased by 0.0006 copies/mL (p=<0.0001), and the estimated odds of medication adherence of adult HIV infected patients was increased by 0.1% (p=0.0046) given other predictors were constant.
As patient’s baseline hemoglobin (gm/dL) increased by one unit, the expected viral load for adult HIV infected patients was decreased by 0.059 copies/mL (p=0.0022), and the estimated odds of medication adherence of adult HIV infected patients was increased by 11% (p=0.0281) given other predictors were constant.
The correlation of random effect between viral load and medication adherence was −0.7688, this leads to a high negative correlation between viral load and medication adherence. This implies that a decrease in viral load tends to increase the chance of good medication adherence.
Discussion
The predictor visit time was significantly associated with viral load. The result showed that as visit time (in months) increases patient’s viral load decreases. This result was consistent with the study done by Kemp et al.20 The result showed that patient’s viral load was decreased as the visit time of patients increase. Moreover, visit time was a statistically significant predictor for medication adherence. The result showed that a one unit increase in visit time (in months) of patients increased the odds of good medication adherence (that is, the increment of visit time of adult HIV infected patients was associated with good medication adherence). This result was in line with the study done by Gelb et al.21 The result showed that there was a positive association between good medication adherence and visit time of adult HIV infected patients.
The predictor substance use was a highly significant association with viral load. The results showed that adult HIV infected patients free from substance use had a lower viral load than substance user adult HIV infected patients. This finding was supported by Reidand Dale.22 The result showed that smoker adult HIV infected patients had a higher viral load than non-smoker patients. Also, the predictor substance use was a significant predictor for medication adherence. The result showed that patients free from substance use had increased odds of good medication adherence as compared with patients who were substance users. This result was consistent with a study conducted by Hinkin et al.23 The finding showed that patients who were free from substance use were more likely than substance user patients to have good medication adherence. The result was also consistent with other studies by Malta et al24,25 and Teter et al.
This study showed that baseline CD4 cell count was a significant predictor for viral load. The analysis showed that, as a unit increase in CD4 cell count (cell/mm3) of adult HIV infected patients, the viral load decreased. This result was contradicted with a study conducted by Shokoand Chikobvu.26 The result showed that adult HIV infected patients with a CD4 cell count between 201–500 cells/mm3 had a decrement in their viral load than patients with CD4 cells greater than 500 cells/mm3. This might be due to differences in study area, sample size, and study period. But, this study was consistent with studies conducted by Swindells et al 27,28 and Desta et al. Moreover, the predictor baseline CD4 cell count was also a significant predictor for medication adherence. The finding showed that as CD4 cell counts of adult HIV infected patients increased by one unit (cells/mm3), the odds of good medication adherence was increased (that is, the increment of CD4 cell of adult HIV infected patients was associated with good medication adherence). This finding was concordant with a study conducted by Abrogoua et al.29 Their finding showed that the increment of baseline CD4 cell count led to good medication adherence. This finding is also consistent with a study conducted by Lima et al.30
Conclusion
The results of separate longitudinal analysis for both viral load and medication adherence responses and joint model analyses for both outcomes were displayed. But, in joint model analyses the two longitudinal response variables were highly correlated and there was a significant reduction in standard error estimates of parameters compared with the separate models. Then more valid inference can be made through joint model analysis.
The predictor’s substance use, WHO clinical stage, visit time, baseline CD4 cell count, marital status, baseline hemoglobin, and the interaction of time by substance use were significant predictors for viral load. And tvisit time, substance use, social support, baseline hemoglobin, baseline CD4 cell count, and the interaction of visit time by substance use were significant predictors for medication adherence.
There was a statistically significant high negative association between viral load and medication adherence. Visit time, substance use, baseline CD4 cell, baseline hemoglobin, and the interaction of visit time by substance use were significant predictors for viral load and medication adherence jointly.
For this investigation substance user adult HIV/AIDS patients and adult HIV/AIDS patients with low baseline CD4 cell and low baseline hemoglobin were with high viral load and poor medication adherence.
Abbreviations
AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; AIDS, Acquired Immune Deficiency Syndrome; FHCSH, Felege Hiwot Comprehensive Specialized Hospital; GLMM, Generalized Linear Mixed Model; HIV, Human Immunodeficiency Virus; PLHIV, People Living with HIV; WHO, World Health Organization.
Data Sharing Statement
The data used for the current investigation is available fromthe corresponding author. For further information; the data used under the current investigation are submitted to the journal as Supplementary Material.
Ethics Approval and Consent to Participate
An ethical clearance certificate was obtained from Bahir Dar University Ethics Committee; Ethiopia; with reference number # RCS/1412/2021. We can attach the ethical clearance certificate upon request. Hence; the work reported in the manuscript was performed according to the national and international institutional rules concerning animal experiments; clinical studies and biodiversity rights. Hence; the clinical data collected by the health staff for medication purposes were secondary for the current study; performed in accordance with the relevant guidelines and regulations of Helsinki and the informed consent to participant was waived by the ethics committee of Bahir Dar University; Ethiopia because of the secondary nature of data that the investigators didn’t get the respondents rather the charts of patients were used.
Acknowledgment
The authors acknowledge all the health staffs at Felege-Hiwot Specialized and Teaching hospital for the data they supplied and their consultancy service given on health-related terminologies.
Author Contributions
All authors made a significant contribution to the work reported; whether that is in the conception; study design; execution; acquisition of data; analysis and interpretation; or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-from-profit sectors.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Kim JY, Farmer P, Porter ME. Redefining global health-care delivery. Lancet. 2013;382(9897):1060–1069. doi:10.1016/S0140-6736(13)61047-8
2. Shahzad M, Chen H, Akhtar T, et al. Human immunodeficiency virus: the potential of medicinal plants as antiretroviral therapy. J Med Virol. 2022;94(6):2669–2674. doi:10.1002/jmv.27648
3. Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status; inequities; and data gaps. J Acquir Immune Defic Syndr. 2014;66:S144–S153. doi:10.1097/QAI.0000000000000176
4. Mohammed H, Assefa N, Mengistie B. Prevalence of extrapulmonary tuberculosis among people living with HIV/AIDS in sub-Saharan Africa: a systemic review and meta-analysis. HIV/AIDS. 2018;10:225.
5. Kärblane K-G. The Effectiveness of International Organisations in the Fight Against HIV/AIDS. Tartu Ülikool; 2018.
6. Alemie GA, Gebreselassie F. Common types of tuberculosis and co-infection with HIV at private health institutions in Ethiopia: a cross sectional study. BMC Public Health. 2014;14(1):1–5. doi:10.1186/1471-2458-14-319
7. Zenebe J. Survival status of undernutrition and its predictors among children who are on antiretroviral therapy in Bahir Dar Town; North West Ethiopia; 2021.
8. Agegnehu CD, Merid MW, Yenit MK. Incidence and predictors of virological failure among adult HIV patients on first-line antiretroviral therapy in Amhara regional referral hospitals; Ethiopia: a retrospective follow-up study. BMC Infect Dis. 2020;20(1):1–14. doi:10.1186/s12879-020-05177-2
9. Mbengue MAS, Chasela C, Onoya D, et al. Clinical predictor score to identify patients at risk of poor viral load suppression at six months on antiretroviral therapy: results from a prospective cohort study in Johannesburg; South Africa. Clin Epidemiol. 2019;11:359. doi:10.2147/CLEP.S197741
10. Rawizza HE, Chaplin B, Meloni ST, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. doi:10.1093/cid/cir729
11. May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193. doi:10.1097/QAD.0000000000000243
12. Ayele W, Mulugeta A, Desta A, et al. Treatment outcomes and their determinants in HIV patients on Anti-retroviral treatment program in selected health facilities of Kembata and Hadiya zones; southern nations; nationalities and peoples region; Ethiopia. BMC Public Health. 2015;15(1):1–13. doi:10.1186/s12889-015-2176-5
13. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:1–12. doi:10.1155/2015/217047
14. Angelo AT, Alemayehu DS. Adherence and its associated factors among adult HIV-infected patients on antiretroviral therapy in South Western Ethiopia; 2020. Patient Prefer Adherence. 2021;15:299. doi:10.2147/PPA.S298594
15. Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV‐infected individuals failing second‐line antiretroviral therapy in Johannesburg; South Africa. Trop Med Int Health. 2016;21(9):1131–1137. doi:10.1111/tmi.12741
16. Cochran W. Sampling Techniques.
17. Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–1192. doi:10.1176/ps.2007.58.9.1187
18. Williams A, Low JK, Manias E, et al. Trials and tribulations with electronic medication adherence monitoring in kidney transplantation. Res Social Adm Pharm. 2016;12(5):794–800. doi:10.1016/j.sapharm.2015.10.010
19. Liu L, Strawderman RL, Cowen ME, et al. A flexible two-part random effects model for correlated medical costs. J Health Econ. 2010;29(1):110–123. doi:10.1016/j.jhealeco.2009.11.010
20. Kemp CG, Lipira L, Huh D, et al. HIV stigma and viral load among African-American women receiving treatment for HIV. AIDS. 2019;33(9):1511–1519. doi:10.1097/QAD.0000000000002212
21. Gelb SR, Shapiro R, Thornton W. Predicting medication adherence and employment status following kidney transplant: the relative utility of traditional and everyday cognitive approaches. Neuropsychology. 2010;24(4):514. doi:10.1037/a0018670
22. Reid R, Dale SK. Moderating effects of social support on the relationship between substance use disorders and HIV viral load and medication adherence among Black women living with HIV in the United States. AIDS Care. 2021;2021:1–10.
23. Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age; cognitive status; and substance abuse. AIDS. 2004;18(Suppl 1):S19. doi:10.1097/00002030-200401001-00004
24. Malta M, Strathdee SA, Magnanini MMF, et al. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–1257. doi:10.1111/j.1360-0443.2008.02269.x
25. Teter CJ, Falone AE, Bakaian AM, et al. Medication adherence and attitudes in patients with bipolar disorder and current versus past substance use disorder. Psychiatry Res. 2011;190(2–3):253–258. doi:10.1016/j.psychres.2011.05.042
26. Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19(1):1–10. doi:10.1186/s12879-019-3781-1
27. Swindells S, Jiang H, Mukherjee AL, et al. Lower CD4 cell count and higher virus load; but not antiretroviral drug resistance; are associated with AIDS-defining events and mortality: an ACTG Longitudinal Linked Randomized Trials (ALLRT) analysis. HIV Clin Trials. 2011;12(2):79–88. doi:10.1310/hct1202-79
28. Desta AA, Wubayehu Woldearegay T, Berhe AA, et al. Immunological recovery; failure and factors associated with CD-4 T-cells progression over time; among adolescents and adults living with HIV on Antiretroviral Therapy in Northern Ethiopia: a retrospective cross sectional study. PLoS One. 2019;14(12):e0226293. doi:10.1371/journal.pone.0226293
29. Abrogoua DP, Kablan BJ, Kamenan BA, et al. Assessment of the impact of adherence and other predictors during HAART on various CD4 cell responses in resource-limited settings. Patient Prefer Adherence. 2012; 6:227.
30. Lima VD, Harrigan R, Murray M, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS. 2008;22(17):2371–2380. doi:10.1097/QAD.0b013e328315cdd3
[ad_2]
Source link

